Luna PFP(2) HPLC Columns

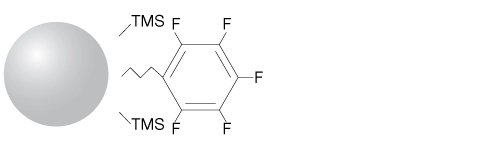
The Luna brand of columns and media is more than just a product line from Phenomenex. It is a pledge to provide you with the highest level of satisfaction for your chromatographic goals. The Luna PFP(2) is a pentafluorophenyl phase with strongly electronegative fluorine groups to provide polar selectivity for cationic compounds, while the rigid bonded phase is a good steric selector.
- Extremely discerning for halogenated‚ aromatic and conjugated compounds
- Provides orthogonal selectivity even using traditional reversed phase mobile phase systems
- PFP Ultra pure, metal-free silica (99.99 % purity) (USP: L43)/li>
Surface Area (m²/g) 400 |
Particle Size 3, 5 |
Recommended Use Separation of aromatic, halogenated, and closely related compounds |
Long Column Lifetimes and Excellent Performance
Superior Particle Smoothness
4 Mechanisms of Interaction
Luna® PFP(2) columns provide unique selectivity for highly polar compounds, complex natural products, isomers and other closely related compounds. This is achieved by using a propyl linked pentafluorophenyl, which provides multiple retention mechanisms unique to typical reversed phase medias.
- Hydrogen Bonding
- Dipole-Dipole Interactions
- Aromatic and π-π Interactions
- Hydrophobic
A typical alkyl phase (C18‚ C8) achieves selectivity through only 1 mechanism of interaction.
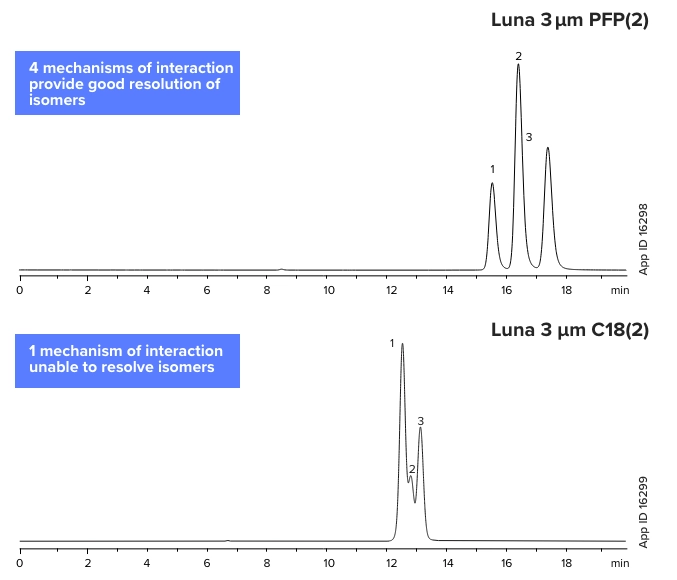
Isomeric Compounds
Positional Isomers of Methylacetophenone
Luna 3 µm PFP(2)
|
Column:
Luna 3 µm PFP(2)
Luna 3 µm C18(2) |
|
Dimensions
150 x 4.6 mm
|
|
Part No.:
|
|
Mobile Phase:
Water/Methanol (50:50)
|
|
Flow Rate:
1 mL/min
|
|
Temperature:
22 °C
|
|
Detection:
UV @ 254 nm
|
|
Sample:
1. o-Methylacetophenone
2. m-Methylacetophenone
3. p-Methylacetophenone
|
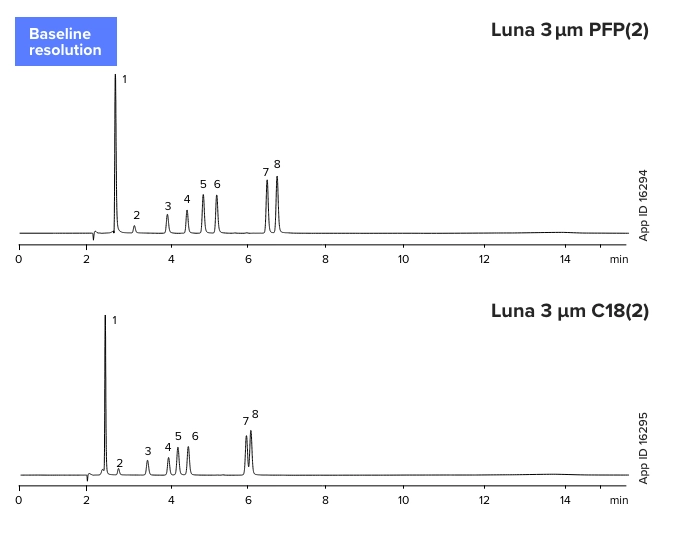
Aromatic Compounds
Catechins
Luna 3 µm PFP(2)
|
Column:
Luna 3 µm PFP(2)
Luna 3 µm C18(2) |
|
Dimensions
150 x 4.6 mm
|
|
Part No.:
|
|
Mobile Phase:
A: 0.1 % Formic acid in Water
B: 0.1 % Formic acid in Acetonitrile
|
|
Gradient:
A/B (80:20) to (55:45) in 10 min
|
|
Flow Rate:
1 mL/min
|
|
Temperature:
22 °C
|
|
Detection:
UV @ 280 nm
|
|
Sample:
1. Gallic acid
2. Epigallo catechin
3. Catechin
4. Epicatechin
5. Epigallocatechin gallate
6. Gallocatechin gallate
7. Epicatechin gallate
8. Catechin gallate
|












